WASHINGTON POST: Inside the clinic where AI and robots are creating babies without human hands
WASHINGTON POST: Robots are learning to make human babies. Twenty have already been born.
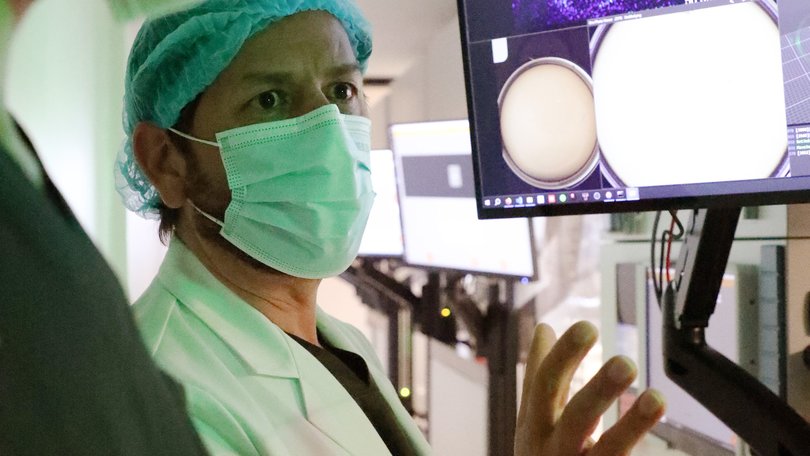
When she walked into an IVF clinic in June, Alin Quintana knew it would be the last time she would try to conceive a child. She had prepared herself spiritually and mentally for the visit: She had travelled to a nearby state to leave prayer ribbons at a shrine dedicated to Mary Undoer of Knots, a manifestation beloved in Latin America. She had received a blessing from her priest.
That morning, as Quintana and her husband wound their way for hours from the unpaved outskirts of the Mexican capital toward the swanky clinic in one of its richest neighbourhoods, her mind was alert, as if she were making a recording. She was focusing on the moment, she said, to drown out the weight of painful memories - a lost pregnancy, a burst fallopian tube, emergency surgery, 96 monthly cycles of hope and crushing disappointment.
But now she was ready to be part of a generational experiment: A robot would create her baby.
Sign up to The Nightly's newsletters.
Get the first look at the digital newspaper, curated daily stories and breaking headlines delivered to your inbox.
By continuing you agree to our Terms and Privacy Policy.
Chatbots have invaded our conversations and self-driving cars roam our streets, but the creation of life is a new frontier for artificial intelligence. IVF has produced more than 13 million babies since its inception in the late 1970s, but it remains a highly manual process. Medical specialists move from station to station in a lab, aspirating tiny egg follicles from bloody, debris-strewn fluid and picking the best sperm swimming in a petri dish with the help of a high-powered microscope.
Using a needlelike pipette, they push the sperm into the centre of an egg many times smaller than a grain of rice. The process, known as intracytoplasmic sperm injection or ICSI, is notoriously delicate; too much force can damage the biological material — one reason IVF, in the best cases, works only about half the time.
Yet quietly, over the past three years, babies have been conceived — and at least 20 of them have been born — through clinical trials that involve automation with little to no human intervention. The same algorithmic computer-vision software that helps autonomous vehicles spot objects on the road and finds signs of breast cancer in a mammogram can instantaneously detect the most robust swimmer among hundreds of thousands of flailing, corkscrewing sperm — each one a fraction of the width of a hair strand. It’s a capability that far exceeds any trained embryologist’s eye. A robotic arm can collect that sperm and mix the chemicals required for an egg to stay viable. And it can delicately and reproducibly fertilise an egg, initiating the moment of conception.
The most ambitious of these automation efforts is happening not in the tech capital of San Francisco, but in Mexico City, at a clinic in the upscale neighborhood of Polanco. There, infertile couples — most of whom live far away and couldn’t afford IVF anyway — are getting automated IVF free in exchange for putting their bodies and their hopes for a family in the robotic hands of an experimental system called Aura.
The trial has potentially global ramifications. One in six people of reproductive age experiences infertility, according to the World Health Organisation. The number is consistent across developed and developing countries and is growing as women delay childbirth and environmental and other factors come into play.
The vast majority of this group — aside from a relatively small percentage in wealthy countries — lacks access to fertility care. Even in the United States, where a single cycle of IVF can cost up to $US30,000 ($46,000) — and most patients require multiple cycles — fertility clinics are concentrated in wealthier coastal cities. Wide swaths of the United States are what researchers call “fertility deserts”.
“Despite the magnitude of the issue,” the WHO concluded in a report in 2023, solutions for the prevention, diagnosis and treatment of infertility “remain underfunded and inaccessible”.
The Aura system, which automates 205 manual steps in IVF from egg freezing to creating an embryo, is made by a start-up called Conceivable Life Sciences. Though headquartered in New York, Conceivable is largely the brainchild of a pioneering Mexican fertility doctor, Alejandro Chávez-Badiola, one of the first physicians to explore how artificial intelligence could be applied to the treatment of infertile patients. Another start-up, Overture Life, which seeks to automate IVF and egg freezing, is already deploying its products commercially in clinics in Turkey and Latin America. None of either companies’ devices are approved by US regulators.

Investors are interested in these technologies for the same reasons the entire corporate world is racing to build AI: Automated systems, which combine robotic hardware and algorithmic software, don’t get tired. They see things the human eye can’t. They can, in theory, do the same work, or at least portions of it, far faster, more precisely and more reliably replicated than human beings can. That means improved results, lower costs and many more patients served.
“Every IVF clinic has its ICSI superstar — the embryologist who gets the best results because they just have that special touch,” said Chávez-Badiola, who is chief medical officer of Conceivable. The superstar phenomenon is one of the reasons he has become a believer in the tech: Performance varies considerably among IVF clinics and even within individual practices. The goal, Chávez-Badiola says, “is to use technology to replicate the superstar every time”.
None of the studies — which include a first-of-its-kind trial in 2023 by Overture and a 2024 study by Conceivable — has demonstrated that AI-enabled machines are markedly outperforming conventional IVF, though researchers have found that the software can excel at key parts of the process, such as picking healthy sperm. And some fertility doctors cautioned that these AI systems remain untested at larger scale and could at least initially introduce costs and burdens on overworked medical providers.
“For any new system … that seems to solve a problem, you probably are taking on another set of problems that sometimes you don’t really understand,” said Serena H Chen, founder and advocacy director at CCRM, a chain of fertility clinics.
But even being on par with an average clinic is a promising early result, said David Sable, a fertility investor, obstetrician and adviser to Conceivable, because it could enable labs to handle more cases, reduce staff fatigue and possibly lower costs over time. “This is about taking the inconsistency humans are prone to and replacing them with the consistency of machines,” he said.
Sable estimates that at least 20 million more babies would be born each year if the industry could meet the global demand from infertile couples, as well as from a growing number of gay families and younger women who want more choices or must freeze their eggs because of medical issues such as cancer.
But fewer than 1 million IVF babies are born annually.
We all know people who would walk on broken glass if I told them they could get pregnant. Right now (IVF) is a concierge marketplace selling high-priced cycles to people that can afford it.
Investor and obstetrician David Sable
A mathematics problem
When Robert G Edwards, the British scientist who invented IVF, won the Nobel Prize, he was honoured for pioneering “a new era in medicine”. But that recognition didn’t come until 2010, 32 years after the first IVF child, Louise Joy Brown, was born to a couple who had been trying to conceive for almost a decade. Back in 1978, Edwards was derided, along with the first cohort of women hoping to become pregnant through this new method. His project was almost scuttled for lack of funding as religious and scientific authorities accused him of manipulating “test tube babies” and playing God.
Today’s cohort in Mexico also deals with stigma. Several couples in the study said in interviews that they did not tell friends or family about their participation. Infertility is a painful and often private experience anywhere, but in Mexico, a predominantly Catholic country, IVF is unfamiliar, out of financial reach and scorned by many people for religious reasons.
“Our church says it has to be natural,” said Noemi Pichardo, 34, who is 22 weeks pregnant using the Aura system. During the couple’s long battle with infertility, “we thought maybe God doesn’t want us to have children,” she said. “That was very hard to accept.”
When a nurse at her gynecologist’s office referred her to the study, she and her husband, Edson Macouzet, did not tell their families. “It’s difficult to explain to people that, in a certain way, AI can do things that a person cannot do,” she said. Now she believes that “maybe IVF is a tool of God”.
Much as Edwards’s first IVF cohort was driven to his experiment by a mix of courage and desperation, the 125 women in the Mexico trial are pioneers of a new technology, Chávez-Badiola said.
The Mexican doctor has a direct line to Edwards. The top student in Edwards’s fertility clinic and lab at Bourn Hall in Cambridge was a Dutch postdoctoral student named Jacques Cohen. Cohen, who went on to run Edwards’s clinic, is credited with inventing or advancing many of the techniques used in embryology labs today, including egg freezing, egg thawing and genetic testing.
Chávez-Badiola left Guadalajara at age 29 in 2005 to go to Liverpool, England, to study reproductive endocrinology. The young physician was drawn to the speciality because it combined “the happiest part of medicine” — creating babies — with an energising technical challenge. Cohen mentored Chávez-Badiola in England and encouraged him to go back to Mexico to start his own clinic, Hope IVF, in Guadalajara. That clinic, and another one in Mexico City, largely catered to the Mexican elite.
Chávez-Badiola still remembers the day in 2016 that started him on his current path. A couple was seeking treatment at the Mexico City clinic. Chávez-Badiola was confident IVF would work for them. They were young and healthy, had no detectable biological markers of infertility, “and their embryos were beautiful,” he recalled.
But the patient went through two IVF cycles and could not get pregnant. So Chávez-Badiola called his team in for an urgent meeting. From now on, he demanded, they were to rigorously analyze their processes, by video recording every embryo the team planned to transfer and counting every single cell in each of them. Doing so was not a common practice in IVF at the time.
A week later, his lab director told him the task was impossible. “He said, ‘The good news is you can actually count the cells,’” Chávez-Badiola recalled. “‘The bad news is it takes ages … and you’re going to drive us all crazy’” with the time demands of the manual count. “The problem,” Chávez-Badiola said, “was math.”
In 2017, Chávez-Badiola sat down with a professor of applied mathematics at the University of Guadalajara. He told the academic that he had thousands of black-and-white images of embryos, each with about 200 cells, and he needed to understand their properties. The professor said: “You’re in luck. Pictures are pixels. Pixels are numbers. And I can do whatever you want with numbers,” he recalled.
Early AI programs called convolutional neural nets, which could teach computers to mark up and recognise objects such as cats and dogs in images, had recently been developed in Silicon Valley. Using those programs, a professor of computer vision Chávez-Badiola had hired, Adolfo Flores Saiffe, created an algorithm that would not only count the embryo cells but also classify their viability, making micromeasurements and detecting subtle properties such as the uniformity of the embryo’s edges. Using what are known as computer vision filters, the program could discern 2.5 million subtle edges and patterns in each photo, 94 of which it deemed medically relevant indicators of a healthy embryo.
Chávez-Badiola’s team’s early papers, the first of which was published in 2020, showed how algorithmic computer vision filters could outperform doctors by 11.6 percent in predicting which embryos were genetically healthy. A subsequent paper predicted which had a chance of miscarriage.
Then the team moved on to sperm, which are particularly challenging to track for doctors and machines because they are “always moving and bumping into one another,” Chávez-Badiola said. One of the engineers he hired had built an AI system to reproduce soccer plays by taking in data feeds from different cameras, and the team applied it to tracking the fast-moving sperm. The AI detected a particular movement — a fast-jerking corkscrew — that hadn’t been previously identified as a sign of a healthy sperm. The team showed, in peer-reviewed research, that a computer could outperform an embryologist in selecting the best sperm, increasing development of embryos ready for implantation by 12 percent.
But one aspect of the AI system remained puzzling, Chávez-Badiola said. Even when it accurately predicted a healthy embryo, it wasn’t always clear why the system made the choice that it did. The software was doing a sophisticated statistical analysis, weighing factors too numerous and subtle for a human brain to process. “That’s where it can become a black box,” he said.
In early 2020, Chávez-Badiola happened to sit next to an entrepreneur named Alan Murray at a dinner in New York. Murray and his co-founder Joshua Abram ran TMRW Life Sciences, a Food and Drug Administration-approved robotic egg storage start-up backed by Silicon Valley investor Peter Thiel.
The men got to talking, and they kept talking during the pandemic. They were brainstorming how to combine the software “brains” Chávez-Badiola had built with TMRW’s hardware expertise.
The men co-founded Conceivable in 2022 and have since raised $US70 million from investors. They started by breaking down IVF into its component parts, from egg retrieval to the “transfer day” when the embryo is implanted in a woman’s uterus. They identified the 205 steps and set about developing technology that could replace each of them. High-precision robotic arms, used to etch electronic patterns onto chips in the semiconductor industry, were deployed to manipulate the biological material as precisely as possible.
At the Polanco clinic, a journey takes place that a Conceivable executive describes as the egg going from its “spa day” in the incubator to its “cold plunge” as an embryo: One station mixes chemicals to prepare the egg for incubation and governs a “smart” incubator to keep it warm. Another robotic station selects and extracts the best sperm, which is then moved to the ICSI station and on to freezing. A small robot — a “handler” — slides back and forth next to the stations, moving petri dishes between them.
An observer who didn’t know that this array of robots was fertilising embryos might mistake it for a smartphone assembly line, with sleek technology making the messiness of biology feel controlled and contained.
After the team built the robotic system, they tested it on mice and produced three generations of healthy pregnancies in the tiny mammals. Cohen, who had joined Conceivable as chief scientific officer, watched these conceptions from a video monitor in New York, ready to stop the process if the robot erred.
They learned by watching. For instance, it takes two pulses from a laser to immobilise the sperm; a single one just wasn’t doing the job to prepare the jumpy male agent for injection into the egg. In early iterations of testing, the sperm sometimes was getting stuck in the pipette and not implanting. The team studied hours of videos of embryologists doing the ICSI process and noticed a subtle shake the lab technicians used to get the sperm out before quickly withdrawing the pipette. So they added code to the algorithm to make the robotic arms do the same little shake.
By 2023, they were ready to start human trials.
Who gets to have a baby?
Just as the Conceivable trial was beginning, Overture Life was finishing its own pilot in New York — using an AI-enabled robot whose software was also trained on hours of real lab footage and had conceived generations of rabbits, hamsters and mice.
That first-of-its-kind pilot yielded results that were about the same as a manual process: The robot produced 13 healthy embryos out of 14 eggs it attempted to fertilise, compared with 16 of 18 fertilising correctly in the manual control group.
One of the three subjects who ultimately received the embryos now has a 2½-year-old daughter, probably the first baby to ever have been born with a robotic assist. The woman, who shared only her first name, Linda, to protect her family’s privacy, said she was terrified to go through the process at first. She was afraid her child might be born with developmental issues or autism. But after six years of trying for a baby, and already in her mid-40s, she was grateful to be accepted into a study that offered her free donor eggs.
None of those concerns remotely trouble her today.
When you see her, you’re like, ‘Wow, she’s so normal! She’s so smart!’ I don’t even remember that my daughter is made with a machine.
But Overture CEO Hans Gangeskar said he realised that robotic fertilisation, however successful, was only part of the puzzle he was trying to solve to make IVF more accessible — and not the most urgent part, either.
Gangeskar keeps a map of the United States by his desk that shows all of America’s “fertility deserts”. Wyoming, for instance, like many rural areas, has fertility clinics where patients can see a doctor, but the closest embryology lab where the actual fertilisation takes place is in nearby Colorado. In rural areas, a woman seeking IVF has many long drives ahead.
And she faces big expenses too, starting with the costly medications to stimulate egg production. Embryology laboratories cost several million dollars to build, and US medical schools train relatively few specialists in reproductive endocrinology and infertility, or REIs, who run them and are highly paid.
In other countries, gynecologists do most of the work of fertility care. That’s one reason 12 percent of births in Spain, where Overture was founded, are generated by IVF, compared with under 3 percent in the U.S. The Spanish government also subsidises the procedure, which costs about $US5000 to $US9000 per cycle.
To address this scarcity, Gangeskar’s team began focusing on designing portable products that can take IVF out of an embryology laboratory altogether. His team has built a palm-size robotic box, called a DaVitri machine, that the company is already shipping to obstetricians all over the world. Ob-gyns in Panama, Brazil, Argentina and Chile — where DaVitri is approved by regulators — retrieve eggs from women during medical visits. With the press of a button, the DaVitri prepares the eggs for freezing by artfully combining cryoprotectant liquids.
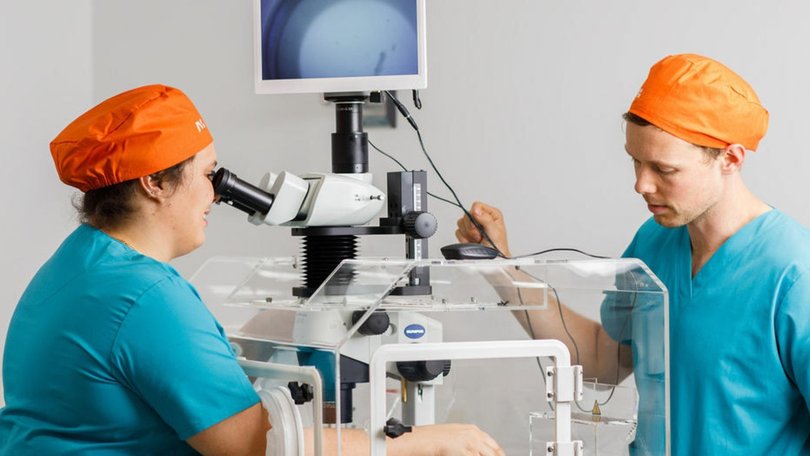
The goal is to take repetitive work — typically done by a highly paid embryologist overseen by an even more highly paid REI in an expensive lab — and compress it into only 15 minutes in a local doctor’s office with virtually no effort required from the doctor. According to Overture’s peer-reviewed studies, 98.3 percent of DaVitri’s eggs survive when thawed, compared with 96.4 percent with standard processes. And the eggs yield 12 percent more embryos ready for implantation, known as blastocysts.
Gangeskar expects to begin selling the DaVitri boxes in the European Union very soon, when Overture’s robotic ICSI machine will begin operating in a large hospital in Istanbul. The company is waiting for approval for DaVitri from the FDA, which has experienced cuts under the Trump administration.
Neither Conceivable nor Overture is saying how much they will charge for their machines. Overture says it costs the company less than $US1000 to make the egg-freezing box. “Our North Star is bringing down the cost,” Gangeskar said.
Sable, the fertility investor, says that the different theories on cost savings are “all going to have to be vetted in the marketplace.”
A rare opportunity
Alin Quintana and her husband, Alejandro Aguilar, both 34, have a chance few people in their economic situation ever get. They work at a family-owned company and make $US1000 combined a week. IVF in Mexico could cost them more than a year’s salary.
They felt extremely lucky even getting into the Conceivable study, and their hopes have since blossomed. A few days after her embryo transfer in June, Quintana found out she was pregnant. She is now 17 weeks along.
She carries the scars of an ectopic pregnancy in 2020, which led to emergency surgery and the removal of one of her fallopian tubes. Though the surgery significantly lowered her chances of becoming pregnant, the couple couldn’t afford fertility care, so they tried naturally for five more years without success. Quintana fell into a deep depression watching as friend after friend got pregnant.
She and Aguilar say they will tell their future child the story of the pregnancy, including the role they hope to have played in helping other people have their own families through automation. The couple is planning to one day give their child a book documenting every step of the process.
“I will say that you were one of the first,” Quintana said. “That we sought you out, with great difficulty. That you were the most wanted baby.
Originally published as Robots are learning to make human babies. Twenty have already been born.
